GETTING TO WORK FAST WITH EYSUVIS®
(loteprednol etabonate ophthalmic suspension) 0.25%
EYSUVIS® provided rapid improvement in ocular discomfort, with results as early as day 4 after starting treatment1
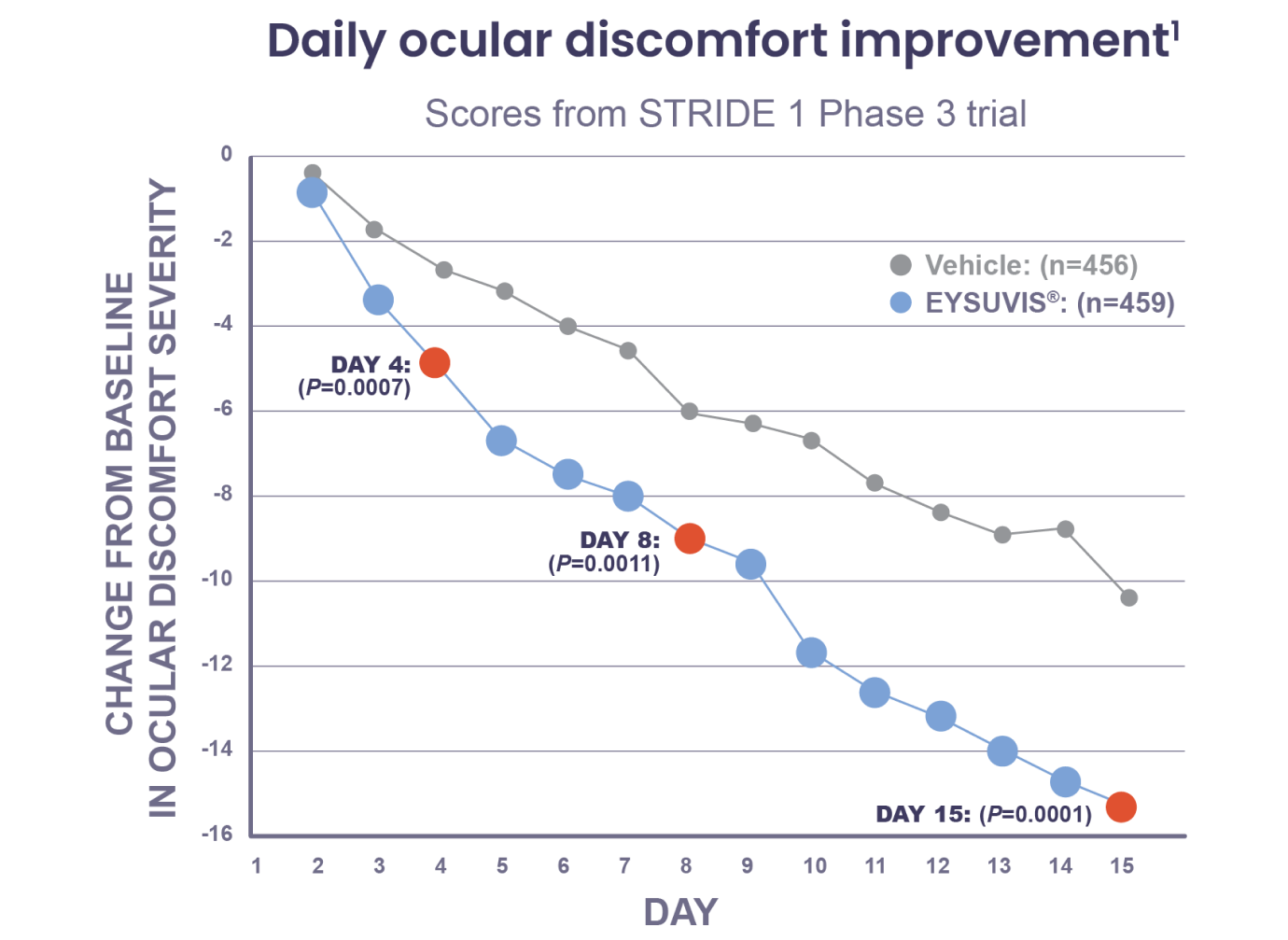
Note: Days 4, 8, and 15 were prespecified endpoints. Inverted scale; lowering of Ocular Discomfort Severity Score from baseline indicates improvement.1
Study Design
The safety and efficacy of EYSUVIS® was assessed in 4 multicentered, randomized, double-masked, placebo-controlled trials (one phase 2 and three phase 3 trials, STRIDE 1, STRIDE 2, and STRIDE 3) in 2871 patients with documented dry eye.2 Patients received either EYSUVIS® or vehicle 4 times a day for at least 2 weeks.2 Day 8 and Day 15 were prespecified efficacy endpoints in STRIDE 1, STRIDE 2, and STRIDE 3; Day 4 was also a prespecified endpoint for STRIDE 1.1 P values for Day 8 and Day 15 were analyzed on the days following Day 7 and Day 14 using the 3-day mean prior to Day 8 (Days 5, 6, and 7) and the 3-day mean prior to Day 15 (Days 12, 13, and 14) compared with the 3-day mean prior to Day 1 (baseline).1 The P value for Day 4 was analyzed using the single-day data compared with the 3-day mean prior to Day 1 (baseline).1 The daily ocular discomfort change from baseline data presented here are derived by comparing the single-day data from each time point to the 3-day mean prior to Day 1 (baseline).1
EYSUVIS® also delivered significant improvements in the reduction of conjunctival hyperemia, an important indicator of inflammation1,3
Conjunctival hyperemia: treatment difference at Day 15 vs vehicle1
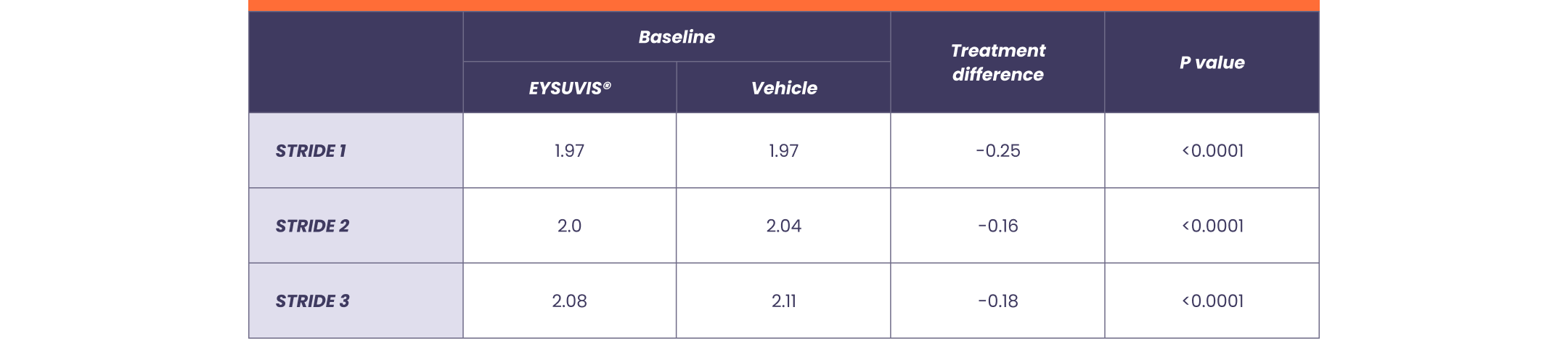
Note: Conjunctival hyperemia was a prespecified primary endpoint in STRIDE 1 and STRIDE 2 and a prespecified secondary endpoint in STRIDE 3.1 STRIDE 1: EYSUVIS® (n=459), Vehicle (n=456). STRIDE 2: EYSUVIS® (n=452), Vehicle (n=453). STRIDE 3: EYSUVIS® (n=447), Vehicle (n=454).1
SD = standard deviation.
REFERENCES
1. Holland E, Nichols K, Foulks G, et al. Safety and efficacy of KPI-121 ophthalmic suspension 0.25% for dry eye disease in four randomized controlled trials. Abstract presented at: AAO 2020; virtual meeting; November 13-15, 2020. Abstract 30064820.
2. Korenfeld M, Nichols KK, Goldberg D, et al. Safety of KPI-121 ophthalmic suspension 0.25% in patients with dry eye disease: a pooled analysis of 4 multicenter, randomized, vehicle-controlled studies. Cornea. 2021;40(5):564-570.
3. Perez VL, Stern ME, Pflugfelder SC. Inflammatory basis for dry eye disease flares. Exp Eye Res. 2020;201:108294.
IMPORTANT SAFETY INFORMATION
Contraindications
EYSUVIS®, as with other ophthalmic corticosteroids, is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures.
INDICATION
EYSUVIS® is a corticosteroid indicated for the short-term (up to two weeks) treatment of the signs and symptoms of dry eye disease.
Warnings and Precautions
Delayed Healing and Corneal Perforation: Topical corticosteroids have been known to delay healing and cause corneal and scleral thinning. Use of topical corticosteroids in the presence of thin corneal or scleral tissue may lead to perforation. The initial prescription and each renewal of the medication order should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and, where appropriate, fluorescein staining.
Intraocular Pressure (IOP) Increase: Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, as well as defects in visual acuity and fields of vision. Corticosteroids should be used with caution in the presence of glaucoma. Renewal of the medication order should be made by a physician only after examination of the patient and evaluation of the IOP.
Cataracts: Use of corticosteroids may result in posterior subcapsular cataract formation.
Bacterial Infections: Use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, corticosteroids may mask infection or enhance existing infection.
Viral Infections: Use of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution. Use of ocular corticosteroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex).
Fungal Infections: Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local corticosteroid application. Fungus invasion must be considered in any persistent corneal ulceration where a corticosteroid has been used or is in use.
Adverse Reactions
The most common adverse drug reaction following the use of EYSUVIS® for two weeks was instillation site pain, which was reported in 5% of patients.
View full Prescribing Information for EYSUVIS®.
